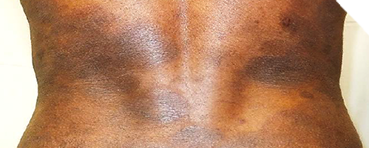
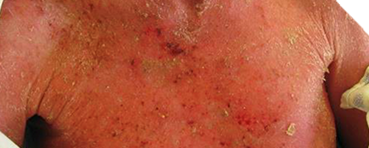
Identifying Potential Patients
Presenting with skin manifestations of CTCL unresponsive to other forms of treatment1
The most common forms of CTCL are mycosis fungoides, which accounts for ~60% of cases, and Sézary syndrome, which accounts for 5% of cases.2 Skin symptoms of mycosis fungoides typically have a long, indolent course.3,4
Patients may experience a long series of different treatments5,8
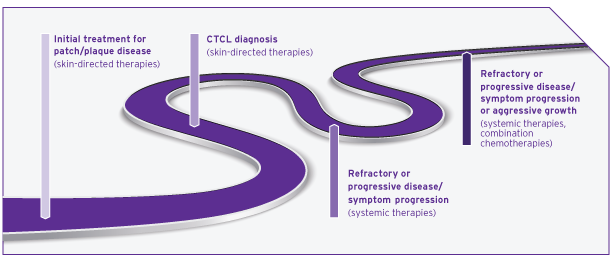
Most patients with skin manifestations of CTCL experience slower progression of their underlying disease than patients with other forms of non-Hodgkin lymphoma (NHL) and should be treated accordingly.6,9
Given the chronic nature of CTCL, tolerability is an important consideration when choosing treatments.10
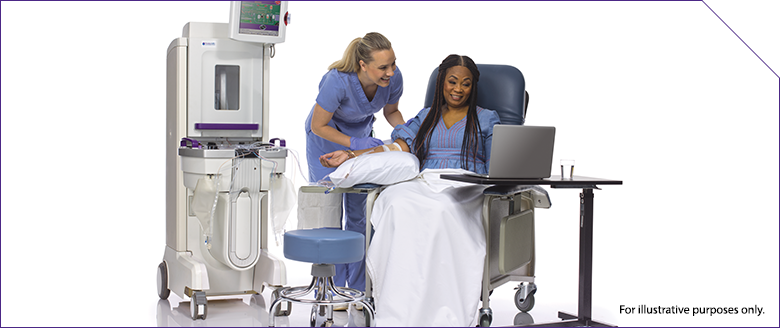
Connecting Patients to Treatment Centers*
THERAKOS Photopheresis is available at most major academic centers
-
Independent treatment centers’ support teams can complement your care
-
Based on your referral, treatment center staff will work with your patient to schedule an intake evaluation and treatments
-
Treatment time: 75 to 105 minutes† per treatment11
Find a local treatment center or contact
our Customer Care team at 877-566-9466
*Treatment centers are independent, third-party facilities not owned or operated by Mallinckrodt Pharmaceuticals
†Depending on use of double- or single-needle mode.
Evaluate Response and Adjust Treatment Schedule if Needed
Evaluate the response
-
The normal treatment schedule is 2 consecutive days every 4 weeks for a minimum of 7 treatment cycles (6 months)1
-
Assess the patient at ~3 months (during the 4th treatment cycle) to determine skin score change from baseline1
-
The 3-month patient assessment can help inform whether to adjust the treatment schedule if indicated1
Adjust the schedule if indicated
-
For patients whose skin score has increased from baseline, consider increasing treatment frequency to 2 consecutive treatments every 2 weeks, which is the accelerated treatment schedule1
-
If a 25% improvement in the skin score is attained after 4 consecutive weeks, the regular treatment schedule may resume1
-
There is no clinical evidence to show that treatment with UVADEX® (methoxsalen) beyond 6 months or using a different schedule provides additional benefit1
Explain your rationale
-
Patients in the clinical study who were unresponsive to the normal treatment schedule were then placed on the accelerated schedule and reassessed for response1
-
Communicating this type of information may help reduce patients’ anxiety, manage expectations, and increase involvement in their own care12
Prepare Your Patients by Setting Expectations
Help them know before they go
Patient adherence to medical recommendations may be impacted through the use of a patient-centered communication style to help build rapport and establish goals. Presenting patients with factual and clear recommendations can increase a patient’s affinity for adherence.13
"For me, improvement happened gradually, which was frustrating. It would be nice to hear ‘you have a clear path.’ But, the truth is that CTCL skin symptoms are unpredictable and not curable."
- THERAKOS Photopheresis patient with CTCL skin symptoms
Photopheresis is not an immediate-response treatment
Clinical response to immunotherapy may take longer to assess than with conventional agents because immunotherapy works by triggering an immune response.14‡ Set expectations that results may come, but it could take at least 3 to 6 months.15
An assessment is appropriate after the fourth treatment cycle, which is approximately 3 months after initiation. The approved course of treatment is a minimum of 7 cycles.1
‡The exact mechanism of action of UVADEX is not known.
"At first, I didn’t think it was working because I didn’t notice any difference in how my skin looked. Other people noticed, but I couldn’t see it. Then, when I went on vacation, I missed a treatment, and I didn’t go for 2 months. Big mistake. I started flaring up. My skin became red and hot, and that chapped and chafed look came back. That’s how I really knew that photopheresis was having an effect."
- THERAKOS Photopheresis patient with CTCL skin symptoms
These minor lifestyle adjustments may help patients get ready
-
Hydrating prior to the procedure in anticipation of fluid shifts.16 Drinking enough water and juice while reducing alcohol and caffeine intake starting 2 days prior to a procedure is preferred
-
Avoiding high-fat foods the night before and on the day of the procedure.16 Low-fat, healthy meals are preferred
"I like that I can work this therapy into my everyday life. During the treatment, I’m able to connect to the Internet and work from my laptop. Sometimes they have to ‘kick me out’ of the room because my treatment is over and I’m still working."
- THERAKOS Photopheresis patient with CTCL skin symptoms
References:
-
UVADEX (methoxsalen) Sterile Solution (prescribing information). Therakos Inc; March 2021.
-
Knobler R, Arenberger P, Arun A, et al. European dermatology forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34(12):2693-2716.
-
Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798-812.
-
Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome. Blood. 2009;114(20):4337-4353.
-
Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer. 2017;77:57-74.
-
Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768-3785.
-
Konstantinow A, Balda B. Treatment of cutaneous T‐cell lymphoma with extracorporeal photochemotherapy. J Eur Acad Dermatol Venereol. 1997;9:111-117.
-
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: T-cell lymphomas. V5.2018. Accessed October 4, 2019. http://www.nccn.org/professionals/physician_gls/
-
Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88(7):2385-2409.
-
Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371(9616):945-957.
-
Data on File — Ref-VV-01420. Mallinckrodt Pharmaceuticals.
-
Prip A, Moller KA, Nielsen DL, et al. The patient-healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs. 2018;41:E11-E22.
-
Lucas AS, Ciccolini K. Nursing best practice referral algorithm for the early detection of mycosis fungoides. J Derm Nurs Assoc. 2016;8(2):109-120.
-
Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805-812.
-
Bisaccia E, Gonzalez J, Palangio M, et al. Extracorporeal photochemotherapy alone or with adjuvant therapy in the treatment of cutaneous T-cell lymphoma: a 9-year retrospective study at a single institution. J Am Acad Dermatol. 2000;43(2 Pt 1):263-271.
-
THERAKOS® CELLEX® Photopheresis System: Operator’s Manual for Use With Software 5.4. 1470493_Rev06_EN-US. Mallinckrodt; 2020.
INDICATIONS AND USAGE
UVADEX® (methoxsalen) Sterile Solution is indicated for extracorporeal administration with the THERAKOS® CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of Cutaneous T-Cell Lymphoma (CTCL) that is unresponsive to other forms of treatment.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
UVADEX is contraindicated in:
-
Patients exhibiting idiosyncratic or hypersensitivity reactions to methoxsalen, other psoralen compounds, or any of the excipients
-
Patients possessing a specific history of a light-sensitive disease state, including lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism
-
Patients with aphakia because of significantly increased risk of retinal damage
-
Patients that have contraindications to the photopheresis procedure
WARNINGS AND PRECAUTIONS
-
Patients who are receiving concomitant therapy (either topically or systemically) with known photosensitizing agents such as anthralin, coal tar or coal tar derivatives, griseofulvin, phenothiazines, nalidixic acid, halogenated salicylanilides (bacteriostatic soaps), sulfonamides, tetracyclines, thiazides, and certain organic staining dyes such as methylene blue, toluidine blue, rose bengal, and methyl orange may be at greater risk for photosensitivity reactions with UVADEX
-
Oral administration of methoxsalen followed by cutaneous UVA exposure (PUVA therapy) is carcinogenic. Methoxsalen also causes DNA damage, interstrand cross-links and errors in DNA repair
-
Methoxsalen may cause fetal harm when given to a pregnant woman. Women of childbearing potential should be advised to avoid becoming pregnant. If UVADEX is used during pregnancy, or if the patient becomes pregnant while receiving UVADEX, the patient should be apprised of the potential hazard to the fetus
-
Severe photosensitivity can occur in patients treated with UVADEX. Advise patients to wear UVA absorbing, wrap-around sunglasses and cover exposed skin or use a sunblock (SPF 15 or higher), and avoid all exposure to sunlight for twenty-four (24) hours following photopheresis treatment
-
After methoxsalen administration, exposure to sunlight and/or ultraviolet radiation may result in "premature aging" of the skin
-
Since oral psoralens may increase the risk of skin cancers, monitor closely those patients who exhibit multiple basal cell carcinomas or who have a history of basal cell carcinomas
-
Serious burns from either UVA or sunlight (even through window glass) can result if the recommended dosage of methoxsalen is exceeded or precautions are not followed
-
Exposure to large doses of UVA light causes cataracts in animals. Oral methoxsalen exacerbates this toxicity
-
Safety in children has not been established
-
Thromboembolic events, such as pulmonary embolism and deep vein thrombosis, have been reported with UVADEX administration through photopheresis systems for treatment of patients with graft-versus-host disease, a disease for which UVADEX is not approved
ADVERSE REACTIONS
-
Side effects of photopheresis (UVADEX used with the THERAKOS Photopheresis Systems) were primarily related to hypotension secondary to changes in extracorporeal volume (>1%)
INDICATIONS
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
The THERAKOS CELLEX Photopheresis System is not designated, sold, or intended for use except as indicated.
Certain underlying medical conditions contraindicate THERAKOS Photopheresis, including patients:
-
who cannot tolerate extracorporeal volume loss during the leukocyte-enrichment phase
-
exhibiting idiosyncratic or hypersensitivity reactions to 8-methoxypsoralen/psoralen compounds
-
with coagulation disorders
-
who have had previous splenectomy
WARNINGS AND PRECAUTIONS
-
THERAKOS Photopheresis treatments should always be performed in locations where standard medical emergency equipment is available. Volume replacement fluids and/or volume expanders should be readily available throughout the procedure
-
Patients who may not be able to tolerate the fluid changes associated with extracorporeal photopheresis should be monitored carefully
-
Procedures, such as renal dialysis, which might cause significant fluid changes (and expose the patient to additional anticoagulation) should not be performed on the same day as extracorporeal photopheresis
-
Individual patients may require a heparin dosage that varies from the recommended dose to prevent post-treatment bleeding or clotting during a treatment
ADVERSE REACTIONS
-
Hypotension may occur during any treatment involving extracorporeal circulation. Closely monitor the patient during the entire treatment for hypotension
-
Transient pyretic reactions, 37.7-38.9°C (100-102°F), have been observed in some patients within six to eight hours of reinfusion of the photoactivated leukocyte-enriched blood. A temporary increase in erythroderma may accompany the pyretic reaction
-
Treatment frequency exceeding labeling recommendations may result in anemia
-
Venous access carries a small risk of infection and pain