Clinical Profile

THERAKOS® Photopheresis delivered a meaningful response in clinical trials1
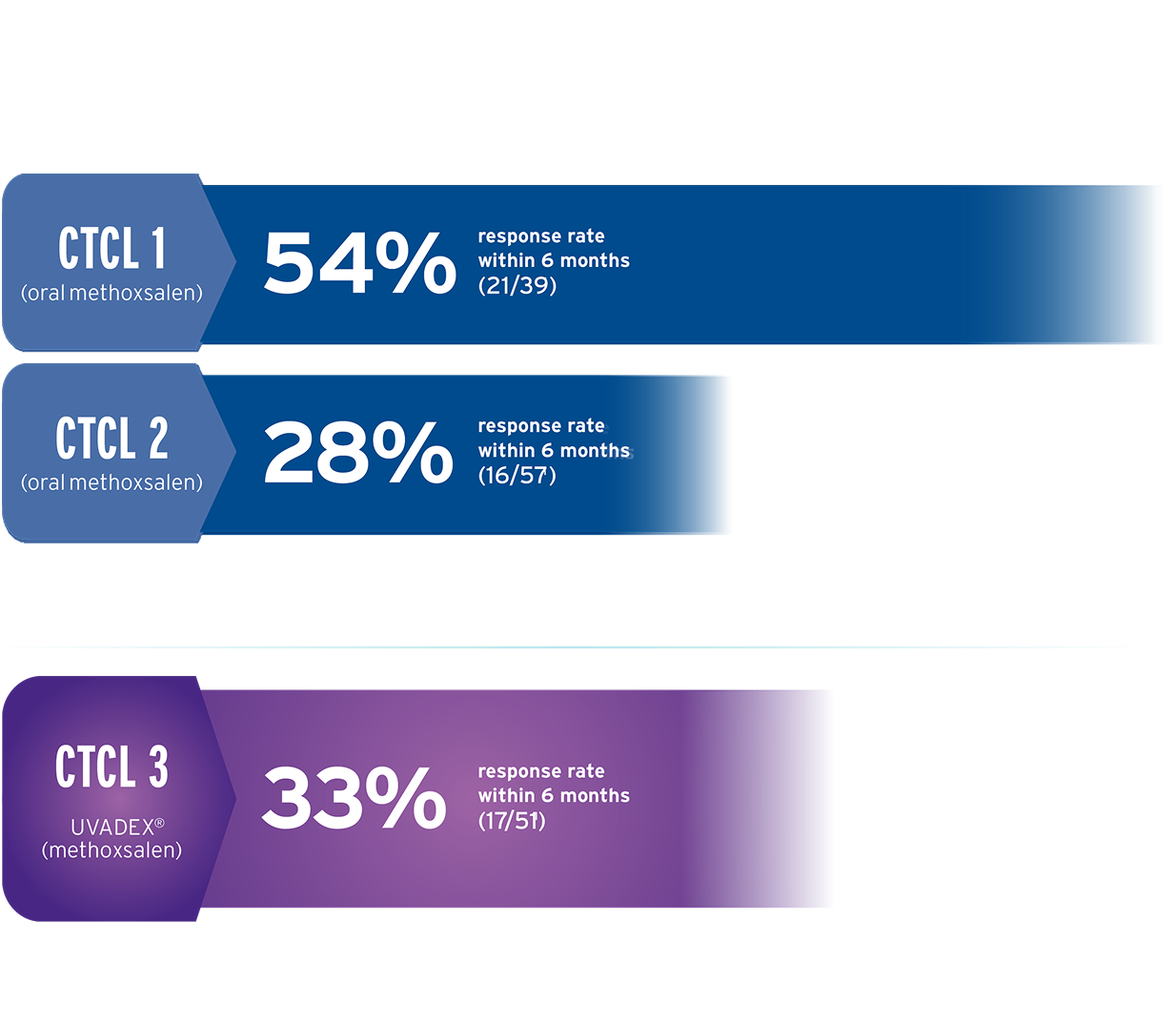
In 3 multicenter, single-arm, open-label trials, efficacy and safety were established in patients (N=147) unresponsive to skin-directed CTCL treatments, including tough-to-treat disease.1,2
A successful treatment response was defined as a ≥25% reduction in overall skin score from baseline maintained for 4 consecutive weeks.1
Study designs
Only patients with patch plaque, extensive plaque, and erythrodermic disease were enrolled in these studies. No patients with disease in the tumor phase were treated.1
CTCL 1 permitted prednisone up to 10 mg/day in addition to topical steroids1
CTCL 2 was a 5-year post approval follow-up to evaluate long-term safety and had no concomitant medication restriction1
CTCL 3, the clinical trial using UVADEX1,2,a
Patients had difficult-to-treat diseaseb and were on an average of 4.3 prior therapies
There are no data available regarding the efficacy of UVADEX in patients with disease in the tumor phase
Patients were only permitted to use topical steroids for the treatment of fissures on the soles of the feet and palms of the hands (all other steroids, topical or systemic, were prohibited)
In CTCL 3:
Of the 17 responses, 15 were observed within 6 months and 2 after 6 months1
Clinical experience does not extend beyond this treatment duration, and there is no evidence to show that treatment with UVADEX beyond 6 months, or using a different schedule, provided additional benefit1
Participants were not required to have blood involvement to qualify for the trials, nor were they screened for it if they met inclusion criteria based on another positive criterion.15,26
Given the chronic nature of ctcl skin symptoms, safety and tolerability are important considerations when choosing treatments3,4
THERAKOS® Photopheresis has been in the market for 35 years with an established safety profile3,1
Adverse events (AEs) in clinical trials were primarily related to hypotension secondary to changes in ECV (>1%)
In the UVADEX® (methoxsalen) clinical trial CTCL 3 (N=51):
6 serious cardiovascular AEs (5 unrelated to Photopheresis) were reported in 5 patients (10%)
6 infections were also reported in 5 patients
2 were Hickman catheter infections in 1 patient
4 infections were not related to Photopheresis
Adverse reactions reported from postmarketing experience included nausea, dysgeusia, photosensitivity reaction, pyrexia, and hypersensitivity reactions, including anaphylaxis and rash
Practicing proper infection control is critical to mitigating the potential for infection.

ECP is recommended by the NCCN Guidelines® as a treatment option for certain patients with CTCL4
References:
1. UVADEX® (methoxsalen) Sterile Solution [prescribing information]. Therakos, Inc. 16. Smith SI, Brodbelt JS. Rapid characterization of cross-links, mono-adducts, and non-covalent binding of psoralens to deoxyoligonucleotides by LC-UV/ESI-MS and IRMPD mass spectrometry. Analyst. 2010;135(5):943-952. doi:10.1039/b924023c 2. Data on File – Ref-05182. Therakos LLC. 3. Knobler R, Arenberger P, Arun A, et al. European dermatology forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34(12):2693-2716. doi:10.1111/jdv.16890 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 26, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 15. UVADEX® (methoxsalen) Sterile Solution [prescribing information]. Therakos, Inc. 26. Data on File – Ref-05182. Therakos LLC.
TKS-25-04816 updated 12/2025







