Prescribing Extracorporeal Photopheresis (ECP) to Treat CTCL Skin Symptoms in Patients Unresponsive to Other Forms of Treatment

Cutaneous T-cell lymphoma (CTCL) is a rare, chronic disease characterized by burdensome skin symptoms2
In the United States, CTCLs constitute 71% of all primary cutaneous lymphomas3,4
The most common types of CTCL are MF (60%) and SS (5%).2

Mycosis fungoides (MF)
Generally a more indolent form of CTCL that usually stays confined to the skin5,6
Characterized by a variety of skin manifestations, including scaly patches, plaques, and nodular lesions2,6
Lesions are often morphologically similar to other inflammatory conditions (eg, eczema, psoriasis) and, together with the slow progression of the disease, can contribute to delayed diagnosis and treatment6

Sézary syndrome (SS)
Patients typically present with erythroderma (>80% BSA), itching, and enlarged lymph nodes5
Commonly characterized by severe itching7
Tends to progress more rapidly than MF and has a worse prognosis7
Patients may experience a long series of different treatments5,8
Most patients with skin manifestations of CTCL experience slower progression of their underlying disease than patients with other forms of non-Hodgkin lymphoma (NHL) and should be treated accordingly.6,9,10 Given the chronic nature of CTCL, tolerability is an important consideration when choosing treatments.8
When patients progress and systemic treatment is next, consider starting with a therapy that is immunomodulatory.7
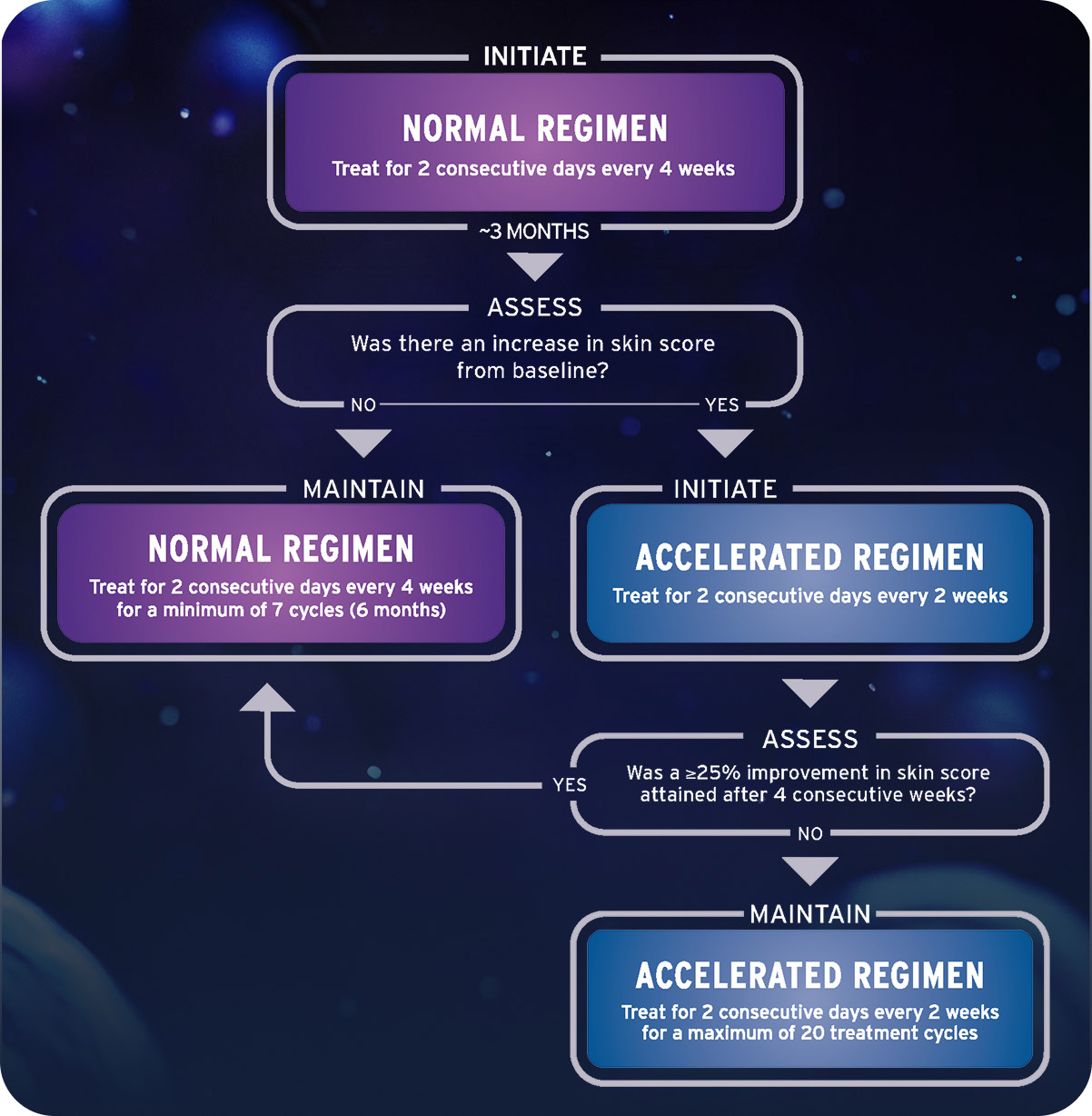
THERAKOS® Photopheresis treatment regimen1
Additional dosing considerations1
Patients in the clinical study who were unresponsive to the normal treatment schedule were placed on the accelerated schedule and reassessed for response. The 3-month patient assessment can help inform whether to adjust the treatment schedule if indicated
Treatment schedule may vary based on individual response and the discretion of the healthcare provider
There is no evidence to show that treatment with UVADEX beyond 6 months or using a different schedule provided additional benefit, and clinical experience does not extend beyond this treatment frequency
Clinical staging of MF and SS4,8


Treatment selection depends on the stage of disease or occurrence of disease progression4
Early-stage MF (IA-IIA) is often managed with skin-directed treatments4
- Topicals
- Phototherapy
- Local radiation
- Total skin electron beam therapy
- Local radiation
- Total skin electron beam therapy
Advanced-stage disease (IIB-IVB) is often managed with systemic treatments4
- Systemic retinoids
- Photopheresis (excluding tumor stage)
- Histone deacetylase inhibitors
- Targeted immunotherapy
- Chemotherapy
- Other
Treatment selection may depend on several factors4,7,9
- Clinical stage (TNMB)
- Morphologic features
- Localization of malignant cells
- Age
- Comorbidities
- Concomitant medications
- Insurance barriers
- Recycling treatments
- Maintenance therapy
- NCCN Guidelines
Prepare Your Patients by Setting Expectations
Help them know before they go
Patient adherence to medical recommendations may be impacted through the use of a patient-centered communication style to help build rapport and establish goals. Presenting patients with factual and clear recommendations can help patients follow their treatment plan.16

Photopheresis is not an immediate-response treatment
Clinical response to immunotherapy may take longer to assess than with conventional agents because immunotherapy works by triggering an immune response.17 Set expectations that results may come, but it could take at least 3 to 6 months.18
An assessment is appropriate during the fourth treatment cycle, which is approximately 3 months after initiation. The approved course of treatment is a minimum of 7 cycles.1

These minor lifestyle adjustments may help patients get ready
Hydrating prior to the procedure in anticipation of fluid shifts.19,20 Drinking enough water and juice while reducing alcohol and caffeine intake starting 2 days prior to a procedure is preferred21,22
Avoiding high-fat foods the night before and on the day of the procedure.15 Low-fat, healthy meals are preferred

Patient information and resources

Connecting Patients to Treatment Centers*
Find a local treatment center or contact our Customer Care team at 1-833-223-4ECP (1-833-223-4327).*Treatment centers are independent, third-party facilities not owned or operated by Therakos LLC.

Patient stories
It may help your patients to hear from people who were treated with THERAKOS® Photopheresis. There are a number of personal stories available that cover a range of experiences that your patients may find relatable.
References: 1. UVADEX (methoxsalen) Sterile Solution (prescribing information). Therakos LLC 2. Knobler R, Arenberger P, Arun A, et al. European dermatology forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34(12):2693-2716. doi:10.1111/jdv.16890 3. Bradford PT, Devesa SS, Anderson WF, Toro JR. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113(21):5064-5073. doi:10.1182/blood-2008-10-184168 4. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Primary Cutaneous Lymphomas V.3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed August 26, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 5. Pulitzer M. Cutaneous T-cell lymphoma. Clin Lab Med. 2017;37(3):527-546. doi:10.1016/j.cll.2017.06.006 6. Yumeen S, Girardi M. Insights into the molecular and cellular underpinnings of cutaneous T cell lymphoma. Yale J Biol Med. 2020;93(1):111-121. 7. Dummer R, Vermeer MH, Scarisbrick JJ, et al. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 2021;7(1):61. doi:10.1038/s41572-021-00296-9 8. Olsen EA, Whittaker S, Willemze R, et al. Primary cutaneous lymphoma: recommendations for clinical trial design and staging update from the ISCL, USCLC, and EORTC. Blood. 2022;140(5):419-437. doi:10.1182/blood.2021012057 9. Olsen EA. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33(4):643-654. doi:10.1016/j.det.2015.06.001 10. Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer. 2017;77:57-74 11. Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768-3785. 12. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Primary Cutaneous Lymphoma. V1.2023. Accessed January 5, 2023. nccn.org 13. Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88(7):2385-2409. 14. Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371(9616):945-957. 15. Prip A, Moller KA, Nielsen DL, et al. The patient-healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs. 2018;41:E11-E22. 16. Lucas AS, Ciccolini K. Nursing best practice referral algorithm for the early detection of mycosis fungoides. J Derm Nurs Assoc. 2016;8(2):109-120. 17. Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11(11):805-812. 18. Bisaccia E, Gonzalez J, Palangio M, et al. Extracorporeal photochemotherapy alone or with adjuvant therapy in the treatment of cutaneous T-cell lymphoma: a 9-year retrospective study at a single institution. J Am Acad Dermatol. 2000;43(2 Pt 1):263-271. 19. THERAKOS® CELLEX® Photopheresis System: Operator’s Manual for Use With Software 5.4. 1470493_Rev06_EN-US. Therakos LLC; 2020. 20. Hoen L, Pfeffer D, Schmidt JR, et al. Hydration status of geriatric patients is associated with changes in plasma proteome, especially in proteins involved in coagulation. Nutrients. 2023;15(17):3789. 21. Montoya GA, Bakuradze T, Eirich M, et al. Modulation of 3',5'-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br J Nutr. 2014;112(9):1427-1437. 22. Sailer CO, Refardt J, Bissig S, et al. Effects of alcohol consumption on copeptin levels and sodium-water homeostasis. Am J Physiol Renal Physiol. 2020;318(3):F702-F709.
TKS-25-04816 updated 12/2025







